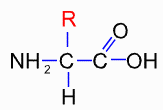
i.e. a central carbon attached to a carboxylate (the COOH), an amino (the NH2) and a hydrogen atom. The differences between the 20 types are in the side groups that can be designated as R. R can either be hydrophobic or hydrophilic.
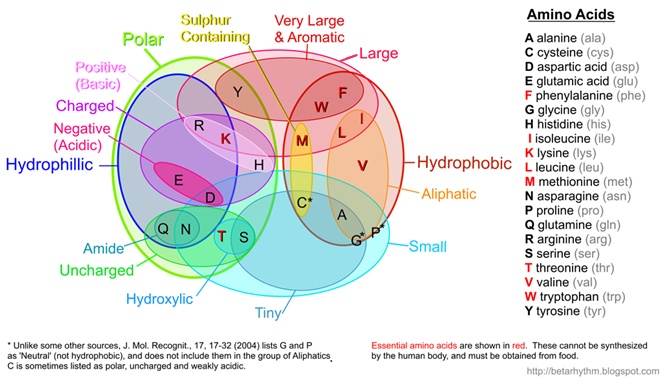 (Note: I've recently updated this image to better align with published results such as Pommié, C. et al., J. Mol. Recognit., 17, 17-32 (2004))
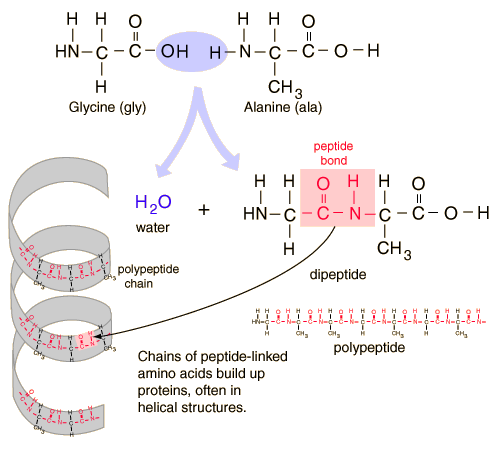
Protein is made by stringing together a bunch of these amino acids with peptide bonds.
(Note: I've recently updated this image to better align with published results such as Pommié, C. et al., J. Mol. Recognit., 17, 17-32 (2004))
Protein is made by stringing together a bunch of these amino acids with peptide bonds.
(Ref: Georgia State University's HyperPhysics site) The body builds around 25000 different types of proteins, some of which act as nano-scale engines (e.g. in muscles), others containing 500-1000 amino acids act as enzymes, some are used as messengers within the cell or between cells, and still others that have both a hydrophobic amino acid side group and a hydrophilic amino acid side group embed themselves in the cell wall and form either ion channels or receptors. (Hey, we're finally getting to the point...) The 3D shape of the protein controls how other molecules interact with the protein. Check out this great flash animation that shows all of the amino acids, the formation of peptide bonds, the formation of alpha helixes and beta sheets and visualization of what it means to 'fold' a protein. Folding of proteins can be examined using 3D Electron Microscopy. "Folds are not "fixed" structures but rather flexible definitions related with the number of secondary structure elements and its spatial distribution." The CATH protein structure classification database catalogs proteins by Class, Architecture, Topology (fold family) and Homologous Superfamily (proteins thought to share a common ancestor).
 Unraveling the Mystery of Protein Folding provides a fascinating overview of recent discoveries about the 3D mechanics of proteins, including the role that improperly folded proteins have been found to play in Alzheimer's disease. IBM's Blue Gene supercomputer is one of the most powerful tools being used in this area of study. It allows researchers to model the 3D forces at play that result in protein folding.
Examples:
Unraveling the Mystery of Protein Folding provides a fascinating overview of recent discoveries about the 3D mechanics of proteins, including the role that improperly folded proteins have been found to play in Alzheimer's disease. IBM's Blue Gene supercomputer is one of the most powerful tools being used in this area of study. It allows researchers to model the 3D forces at play that result in protein folding.
Examples: G Protein-Coupled Receptors (GPCR) in a membrane environment
Lipids critical to cell division and fusion
More... From the excellent Medical Biochemistry page at Indian University: Protein Structure - covers hydrogen bonding and other forces involved in folding proteins. Charge Densities, Hydrogen Bonding and Drug Design shows electron density contributions from lone pair, covalent and hydrogen bond electrons.
No comments:
Post a Comment